Lipid molecules can induce an opening of membrane-facing tunnels in cytochrome P450 1A2†
Abstract
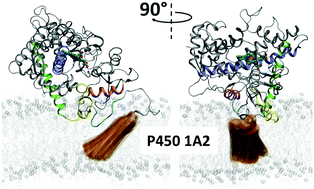
Cytochrome P450 1A2 (P450 1A2, CYP1A2) is a membrane-bound enzyme that oxidizes a broad range of hydrophobic substrates. The structure and dynamics of both the catalytic and trans-membrane (TM) domains of this enzyme in the membrane/water environment were investigated using a multiscale computational approach, including coarse-grained and all-atom molecular dynamics. Starting from the spontaneous self-assembly of the system containing the TM or soluble domain immersed in randomized dilauroyl phosphatidylcholine (DLPC)/water mixture into their respective membrane-bound forms, we reconstituted the membrane-bound structure of the full-length P450 1A2. This structure includes a TM helix that spans the membrane, while being connected to the catalytic domain by a short flexible loop. Furthermore, in this model, the upper part of the TM helix interacts directly with a conserved and highly hydrophobic N-terminal proline-rich segment of the catalytic domain; this segment and the FG loop are immersed in the membrane, whereas the remaining portion of the catalytic domain remains exposed to aqueous solution. The shallow membrane immersion of the catalytic domain induces a depression in the opposite intact layer of the phospholipids. This structural effect may help in stabilizing the position of the TM helix directly beneath the catalytic domain. The partial immersion of the catalytic domain also allows for the enzyme substrates to enter the active site from either aqueous solution or phospholipid environment via several solvent- and membrane-facing tunnels in the full-length P450 1A2. The calculated tunnel dynamics indicated that the opening probability of the membrane-facing tunnels is significantly enhanced when a DLPC molecule spontaneously penetrates into the membrane-facing tunnel 2d. The energetics of the lipid penetration process were assessed by the linear interaction energy (LIE) approximation, and found to be thermodynamically feasible.
- This article is part of the themed collection: Insights from advanced methods in molecular dynamics


 Please wait while we load your content...
Please wait while we load your content...