Infrared and fluorescence assessment of the hydration status of the tryptophan gate in the influenza A M2 proton channel†
Abstract
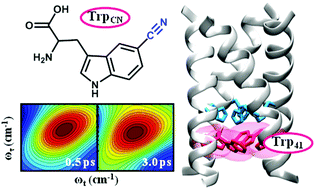
The M2 proton channel of the influenza A virus has been the subject of extensive studies because of its critical role in viral replication. As such, we now know a great deal about its mechanism of action, especially how it selects and conducts protons in an asymmetric fashion. The conductance of this channel is tuned to conduct protons at a relatively low biologically useful rate, which allows acidification of the viral interior of a virus entrapped within an endosome, but not so great as to cause toxicity to the infected host cell prior to packaging of the virus. The dynamic, structural and chemical features that give rise to this tuning are not fully understood. Herein, we use a tryptophan (Trp) analog, 5-cyanotryptophan, and various methods, including linear and nonlinear infrared spectroscopies, static and time-resolved fluorescence techniques, and molecular dynamics simulations, to site-specifically interrogate the structure and hydration dynamics of the Trp41 gate in the transmembrane domain of the M2 proton channel. Our results suggest that the Trp41 sidechain adopts the t90 rotamer, the χ2 dihedral angle of which undergoes an increase of approximately 35° upon changing the pH from 7.4 to 5.0. Furthermore, we find that Trp41 is situated in an environment lacking bulk-like water, and somewhat surprisingly, the water density and dynamics do not show a measurable difference between the high (7.4) and low (5.0) pH states. Since previous studies have shown that upon channel opening water flows into the cavity above the histidine tetrad (His37), the present finding thus provides evidence indicating that the lack of sufficient water molecules near Trp41 needed to establish a continuous hydrogen bonding network poses an additional energetic bottleneck for proton conduction.

 Please wait while we load your content...
Please wait while we load your content...