Synergistic formation of sulfate and ammonium resulting from reaction between SO2 and NH3 on typical mineral dust†
Abstract
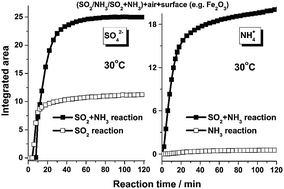
The heterogeneous reactions of SO2 and NH3 on typical mineral oxides were investigated using in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). A new sulfate formation pathway was proposed where NH3 accelerated the formation of sulfate species. The results revealed that surface hydroxyls and oxygen played principal roles in the conversion of SO2 to sulfate. It was proposed that NH3 adsorbed onto Lewis acid sites, and hydroxyls and water molecules adsorbed on the surfaces of mineral dust. The enhancement of surface Lewis basicity by NH3 induced more SO2 molecules to adsorb on the surface, which were further oxidized to sulfate by interacting with surface hydroxyls and oxygen atoms. The formation of sulfate, in turn, contributed to the adsorption of NH3, mainly as NH4+ due to enhanced Brønsted acid sites. The IC results showed that the synergistic effect between SO2 and NH3 was more significant on acidic oxides like γ-Al2O3 and α-Fe2O3 compared to basic oxides like MgO.
- This article is part of the themed collection: Environmental Science: Atmospheres – Introducing our new Advisory Board Members

 Please wait while we load your content...
Please wait while we load your content...