Unification of ground-state aromaticity criteria – structure, electron delocalization, and energy – in light of the quantum chemical topology
Abstract
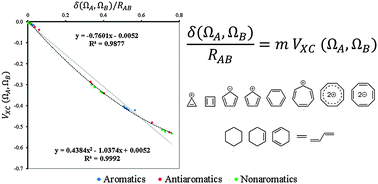
In the present account we investigate a theoretical link between the bond length, electron sharing, and bond energy within the context of quantum chemical topology theories. The aromatic stabilization energy, ASE, was estimated from this theoretical link without using isodesmic reactions for the first time. The ASE values obtained from our method show a meaningful correlation with the number of electrons contributing to the aromaticity. This theoretical link demonstrates that structural, electronic, and energetic criteria of aromaticity – ground-state aromaticity – belong to the same class and guarantees that they assess the same property as aromaticity. Theory suggests that interatomic exchange–correlation potential, obtained from the theory of Interacting Quantum Atoms (IQA), is linearly connected to the delocalization index of Quantum Theory of Atoms in Molecules (QTAIM) and the bond length through a first order approximation. Our study shows that the relationship between energy, structure and electron sharing marginally deviates from the ideal linear form expected from the first order approximation. The observed deviation from linearity was attributed to a different contribution of exchange–correlation to the bond energy for the σ- and π-frameworks. Finally, we proposed two-dimensional energy-structure-based aromaticity indices in analogy to the electron sharing indices of aromaticity.
- This article is part of the themed collection: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

 Please wait while we load your content...
Please wait while we load your content...