Cationic M2L4 cages for perchlorate removal from aqueous solutions and preferential perchlorate incorporation in hydrophilic solutions†
Abstract
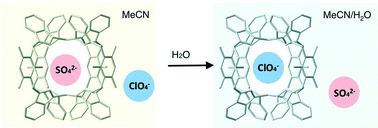
Four new cage-type compounds, [A ⊂ Cu2(m-bbitrb)4]A3 (A = Cl− (1a), Br− (1b), m-bbitrb = 1,3-bis(benzimidazol-1-ylmethyl)-2,4,6-trimethylbenzene), and [SO4 ⊂ Cu2(m-bbitrb)4]A2 (A = Cl− (2a), Br− (2b)), were synthesized and characterized. These compounds have a M2L4-type cationic cage, incorporating an anion in the cage. Although the four compounds are insoluble in water, they exhibit ClO4− removal activity from aqueous solutions by exchange with anions located outside the cage. The [Cl ⊂ Cu2(m-bbitrb)4]3+ cage removes ClO4− from aqueous solutions preferentially. To study the selective removal process by the cationic cage, the affinity of the [Cu2(m-bbitrb)4]4+ cage for ClO4− was studied by acquiring the solid-state reflectance spectra, absorption spectra, and ESI-TOF mass spectra in solutions of MeCN and MeCN/H2O for [SO4 ⊂ Cu2(m-bbitrb)4](ClO4)2 (2d). The results indicated that the SO42− in [SO4 ⊂ Cu2(m-bbitrb)4]2+ was retained in the MeCN solution, whereas in the MeCN/H2O solution the SO42− was exchanged with the ClO4− located outside the cage. This is because an increase in the hydrophilicity of the solution promotes the incorporation of hydrophobic ClO4− into the hydrophobic cage, meaning that the preferential removal of ClO4− from aqueous solutions is due to the higher affinity of the hydrophobic space created outside the M2LB4 cages in the solid state.
- This article is part of the themed collection: Form and Function of Molecular Cups and Capsules

 Please wait while we load your content...
Please wait while we load your content...