Proteomic profile of 4-PBA treated human neuronal cells during ER stress†
Abstract
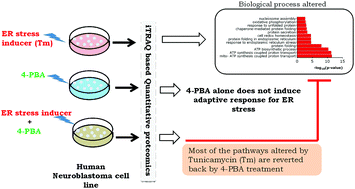
Perturbations affecting the homoeostasis of endoplasmic reticulum (ER) activate an adaptive signaling known as the unfolded protein response or UPR. Many studies have reported the association between neurological disorders and ER stress. Decreasing ER stress may therefore aid in therapeutic control of neuronal diseases. Sodium 4-phenylbutyrate (4-PBA), a small molecule, has been shown to alleviate ER stress and various neurological diseases, but the mechanistic basis of its action is not well understood. Using an iTRAQ based LC-MS technique we have delineated the effect of 4-PBA on the proteome of human neuroblastoma cells (SK-N-SH) during Tunicamycin-induced ER stress. The proteomic profile of 4-PBA-treated cells revealed that 4-PBA does not alter the cellular proteome to adapt towards ER stress. However, it can alleviate both the toxicity and proteomic alterations, induced by an ER stress inducer. Hence, the therapeutic effect of 4-PBA is primarily due to its ability to resolve ER stress rather than its ability to alter the expression of proteins required for maintaining ER proteostasis. Thus, we posit here that 4-PBA acts as an authentic chemical chaperone by aiding protein folding in the ER.


 Please wait while we load your content...
Please wait while we load your content...