A facile strategy to fabricate nitrogen-doped graphene aerogel-supported Fe3N nanoparticles as efficient electrocatalysts for the oxygen reduction reaction†
Abstract
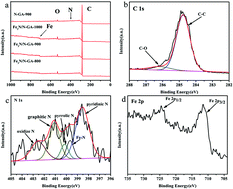
In this study, a novel hybrid composed of iron nitride and nitrogen-functionalized graphene aerogel (Fe3N/N-GA) was fabricated and used as an electrode material for the oxygen reduction reaction (ORR). The Fe3N/N-GA obtained at the pyrolysis temperature of 900 °C, denoted as Fe3N/N-GA-900, exhibits outstanding catalytic activity towards the ORR compared to other Fe3N/N-GA hybrids, which may be due to the highest absolute content of Fe–N and graphitic-N species in the Fe3N/N-GA-900. Remarkably, the fabricated Fe3N/N-GA-900 hybrid is comparable to the benchmark Pt/C catalyst in terms of the onset potential and half-wave potential, but it possesses a larger kinetic energy, which limits the current density, and better methanol tolerance and operational stability than those of the commercial Pt/C catalyst for the ORR in an alkaline medium. Therefore, it holds great promise as a replacement for the Pt/C catalyst in alkaline direct methanol fuel cells (DMFCs).


 Please wait while we load your content...
Please wait while we load your content...