High LUMO energy pyrrolidinofullerenes as promising electron-acceptor materials for organic solar cells†
Abstract
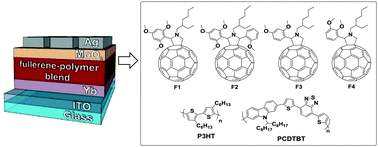
We report the synthesis and investigation of four novel pyrrolidinofullerenes bearing electron donating alkoxyphenyl substituents. Cyclic voltammetry studies revealed that the designed fullerene derivatives exhibit reduced electron affinity compared to the conventional material [60]PCBM. Organic bulk heterojunction solar cells based on pyrrolidinofullerenes and P3HT revealed impressive open-circuit voltages of 700–780 mV and enhanced light power conversion efficiencies with respect to reference PCBM/P3HT devices. The designed materials demonstrated reasonably good compatibility and decent photovoltaic performances in combination with a carbazole–thiophene–benzothiadiazole copolymer PCDTBT.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry C Hot Papers

 Please wait while we load your content...
Please wait while we load your content...