Facile synthesis of porous Fe2TiO5 microparticulates serving as anode material with enhanced electrochemical performances†
Abstract
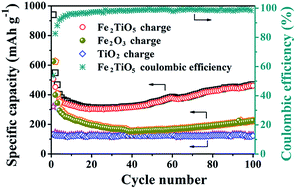
Porous iron titanium oxide (Fe2TiO5) microparticulates have been successfully synthesized via a facile hydrothermal route followed by a subsequent calcination process. Polyvinyl-pyrrolidone (PVP), serving as a surfactant, played a pivotal role in controlling the size and inducing the mesoporous structure of Fe2TiO5. The measured specific surface area is 83.1 m2 g−1 and the dominant pore size is ca. 10 nm. When tested as the anode material of lithium-ion batteries (LIBs), the as-prepared porous Fe2TiO5 microparticulates delivered a high reversible capacity of 468.3 mA h g−1 after 100 cycles at a current density of 100 mA g−1, which nearly quadrupled that of porous TiO2 microspheres and doubled that of Fe2O3 nanoparticles. Moreover, the porous Fe2TiO5 microparticulates also showed more superior rate capability and long-term cycling stability with respect to TiO2 and Fe2O3 samples. Even after the rate performance test, a high discharge capacity of 234.1 mA h g−1 was still maintained at a current density of 500 mA g−1 over another 250 cycles. The improved electrochemical performances are mainly attributed to the synergistic effect of TiO2 and Fe2O3 in Fe2TiO5, as well as the mesoporous structure.

 Please wait while we load your content...
Please wait while we load your content...