Synthesis and evaluation of novel quinuclidinone derivatives as potential anti-proliferative agents†
Abstract
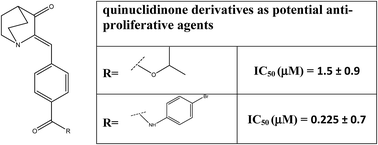
In this study a new series of substituted (Z)-4-(3-oxoquinuclidin-2-ylidene) benzamides and substituted (Z)-4-((3-oxoquinuclidin-2-ylidene)methyl)benzoates have been designed and synthesised as potential anti-cancer agents. This set of compounds were prepared by using a common intermediate (Z)-4-((3-oxoquinuclidin-2-ylidene)methyl)benzoic acid. They were well characterized by various spectroscopic techniques as well as a crystallographic study and screened for anti-cancer activity. A cell viability assay using MTT was performed on A549 & L132 cell lines and IC50 values were determined. Analogues 4c and 5e exhibited the most potent anti-cancer activity among all the analogues synthesized in this present study. A haemolytic assay using normal human erythrocytes was performed to study the blood compatibility of the compounds. Acridine orange/ethidium bromide (AO/EB) staining also showed cell death. To get a better insight into the mechanism of cell death DAPI (4′,6-diamido-2-phenylindol nuclear staining) and DNA fragmentation studies were performed. A Structure Activity Relationship (SAR) was explored to facilitate further development of this new class of compounds.

 Please wait while we load your content...
Please wait while we load your content...