Multifunctional 3-Schiff base-4-hydroxycoumarin derivatives with monoamine oxidase inhibition, anti-β-amyloid aggregation, metal chelation, antioxidant and neuroprotection properties against Alzheimer's disease†
Abstract
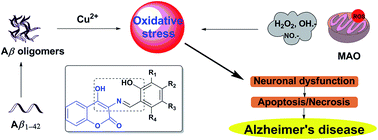
A series of 3-Schiff base-4-hydroxycoumarin derivatives (1–20) have been designed, synthesized and evaluated as multifunctional agents against Alzheimer's disease. In vitro studies indicated that most of the derivatives exhibited significant abilities to inhibit monoamine oxidase (MAO), self-induced and Cu2+-induced β-amyloid (Aβ1–42) aggregati006Fn, as well acting as potential biometal chelators and antioxidants. Moreover, these derivatives were capable of decreasing reactive oxygen species (ROS) and showed good neuroprotective effects in PC12 cells and could penetrate the central nervous system (CNS). In particular, compound 4 exhibited high potency to monoamine oxidase (IC50, 0.673 μM for hMAO-A, 0.711 μM for hMAO-B), good antioxidant activity (1.34 trolox equivalents by ABTS method, 45.8 μM of IC50 by DPPH method), and it also displayed a significant ability to inhibit self-induced and Cu2+-induced Aβ1–42 aggregation (60.1%, 20 μM and 45.7%, 50 μM). Taken together, these results suggested that compound 4 might be a promising lead compound with balanced properties for AD treatment.
- This article is part of the themed collection: Towards understanding and treating Alzheimer’s disease

 Please wait while we load your content...
Please wait while we load your content...