Chemical and electrochemical hydrogenation of CO2 to hydrocarbons on Cu single crystal surfaces: insights into the mechanism and selectivity from DFT calculations†
Abstract
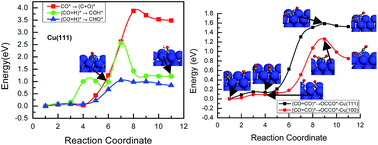
Atomic level mechanistic insights into the chemical and electrochemical reduction of CO2 on the Cu(111) and Cu(100) surfaces are presented based on DFT-based thermodynamic and kinetic calculations. On Cu(111), COads is firstly formed by the dissociative hydrogenation of CO2, and CHOads and CH2Oads are the key intermediates towards the chemical and electrochemical reduction of CO2 into methanol and CH4. Despite being thermodynamically or kinetically favoured, it is likely that CH2OHads instead of CH3Oads is the intermediate for methanol and CH4 formation. Based on the activation barriers, CH2OHads intermediate either forms CH3OH by direct hydrogenation or forms CH2ads by hydrogenative dissociation, which may be a parallel path in the CO2 reduction mechanism on the Cu(111) surface. Finally, the CH2 intermediate leads to formation of the hydrocarbons. On Cu(100), CO2 reduction takes a different pathway in the early stages; CO is formed through the direct dissociation of CO2 rather than hydrogenative dissociation as on the Cu(111) surface due to stronger bonding of CO. Further reduction of CO also undergoes a different pathway, in which CO dimerization is more easy to achieve, whereas CO hydrogenation is difficult on the Cu(100) surface. This explains why C2H4 is formed more favorably on the Cu(100) surface and CH4 is predominantly produced on the Cu(111) surface under both chemical and electrochemical conditions. In addition, DFT results showed for the first time that the electrochemical reduction would be expected to be highly favored at potentials of interest by CO2 reduction compared with chemical reduction and that the carbon dioxide anion radical (˙CO2−) is involved in the initial stage of CO2 electroreduction. Simultaneously, the results also explain partly why CH3OH is formed in gas phase chemistry and only CH4 is observed in electrochemistry on copper surfaces. By analyzing the chemical and electrochemical reduction paths, important mechanistic information is deduced on the Cu single crystal surfaces. The study of CO2 reduction mechanisms on copper will lead to a deeper understanding of the reaction chemistry and could eventually lead to the design of more efficient and selective catalysts.
- This article is part of the themed collection: Nanoscience and nanotechnology in electrochemistry

 Please wait while we load your content...
Please wait while we load your content...