Regioselective palladium(ii)-catalyzed aerobic oxidative Heck-type C3 alkenylation of sulfocoumarins†
Abstract
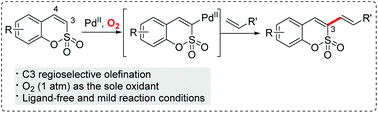
An efficient method for the direct C–H olefination of sulfocoumarins with a wide range of alkenes is developed. Moreover, O2 was successfully utilized as the sole oxidant for the oxidative Heck reaction. This approach enables the rapid generation of various 3-alkenylated sulfocoumarins.
- This article is part of the themed collections: 2015 Emerging Investigators by OCF and HOT articles in Organic Chemistry Frontiers in 2015

 Please wait while we load your content...
Please wait while we load your content...