Speciation and kinetic study of iron promoted sugar conversion to 5-hydroxymethylfurfural (HMF) and levulinic acid (LA)†
Abstract
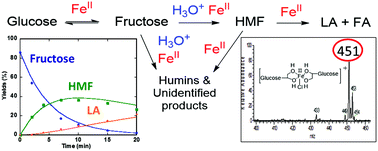
Cellulose, a major component of renewable biomass, is a polymer of glucose. Abundant and cheap iron salts promote the conversion of glucose to 5-hydroxymethylfurfural (HMF) and levulinic acid (LA). In this study, glucose transformations catalyzed by iron(III) chloride (FeCl3) in aqueous and in biphasic media (water and 2-methyltetrahydrofuran (MeTHF)) were investigated. Speciation via mass spectrometry (MS), UV-Vis, and X-ray absorption spectroscopy (XAS) show that FeIII is reduced to FeII (over 95%) readily in the early stage of carbohydrate conversion. The reaction time profiles of reactants (glucose and fructose) as well as products (HMF and LA) were modeled using MATLAB to obtain reaction rate constants. The contributions of iron and the intrinsic Brønsted acidity of iron salts in the sugar conversion are discussed. The kinetic study of sugar conversion indicated that the water–MeTHF biphasic system hinders the conversion of sugars to humins and unknown byproducts and increases the yields of HMF and LA. By adjusting concentrations of FeII and Brønsted acidity, yields of 88% LA (FeCl3, pH = 1) or 56% HMF (FeSO4, pH = 2) from glucose in a water–MeTHF biphasic system are achieved. The optimized reaction conditions proved effective in the conversion of milled poplar biomass to LA (53% yield based on glucose content) and furfural (64% yield based on xylan content) using iron salt, outperforming aluminum and chromium salts.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2015

 Please wait while we load your content...
Please wait while we load your content...