Molecular recognition of upper rim functionalized cavitand and its unique dimeric capsule in the solid state†
Abstract
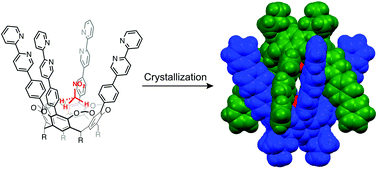
Cavitand 1 possesses four 2,2′-bipyridyl pillars on its upper rim that encapsulates small guests, such as nitromethane, acetonitrile, methyl acetate, ethyl acetate, and N-methylacetamide, into a deep cavity to form host–guest complexes in a 1 : 1 ratio. Nitroethane, N,N-dimethylformamide, and N,N-dimethylacetamide were not bound in this manner. A guest-binding study and molecular mechanics calculations revealed that the four 2,2′-bipyridyl pillars of cavitand 1 created a steric boundary that is responsible for selective guest recognition. In the solid state, cavitand 2 formed a unique chiral capsule 22 by π–π stacking interactions between the 2,2′-bipyridyl pillars. A nitromethane molecule was unusually placed deep inside the cavity, as directed by the multiple hydrogen bonding interactions between the nitromethane oxygen atoms, the C–H bonds of the bridge methylenes and the pillar phenyl groups.
- This article is part of the themed collection: In celebration of Seiji Shinkai's 70th Birthday

 Please wait while we load your content...
Please wait while we load your content...