Polyoxometalate-induced ‘cage-within-cage’ metal–organic frameworks with high efficiency towards CO2 photoreduction†
Abstract
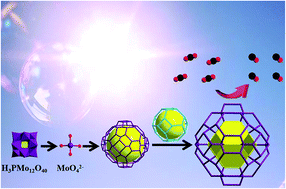
Photocatalytic CO2 reduction (CO2RR) into high-value products is of great significance not only in addressing global warming but also in developing sustainable energy resources. Herein, two novel metal–organic frameworks (MOFs) induced by polyoxometalates (POMs) have been successfully synthesized via a solvothermal method, formulated as {Zn4(MoO4)(HCO2)3(C4H5N2)3}·DMF (Zn/Mo-MOF) and {Zn2Co2(MoO4)(HCO2)3(C4H5N2)3}·DMF (Zn/Co/Mo-MOF) [DMF = HCON(CH3)2]. The single crystal X-ray diffraction (SCXRD) analysis reveals that both MOFs contained MoO42− anions as the building units, which resulted from the decomposition of phosphomolybdic acid (H3PMo12O40). Notably, the cage β in MOFs was constructed by MoO42− anions, and this cage was located outside and interconnected with independent cage α with a sodalite cage-type structure to arrange into a rare cage-within-cage structure. More interestingly, Zn/Co/Mo-MOF exhibited a remarkable CO production rate (38.41 μmol h−1) with a high selectivity of 91.4% for the CO2 photoreduction, which outperforms most of the reported MOF-based photocatalysts. The result of this study demonstrates that the successful introduction of the POM's anions to construct MOFs may open up new opportunities to synthetize highly efficient MOF-based photocatalysts for the CO2 photoreduction.


 Please wait while we load your content...
Please wait while we load your content...