Measuring binding kinetics of aromatic thiolated molecules with nanoparticles via surface-enhanced Raman spectroscopy†
Abstract
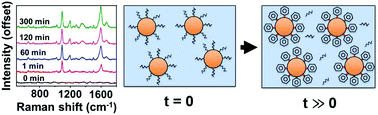
Colloidal plasmonic nanomaterials, consisting of metals such as gold and silver, are excellent candidates for advanced optical probes and devices, but precise control over surface chemistry is essential for realizing their full potential. Coupling thiolated (R-SH) molecules to nanoprobe surfaces is a convenient and established route to tailor surface properties. The ability to dynamically probe and monitor the surface chemistry of nanoparticles in solution is essential for rapidly manufacturing spectroscopically tunable nanoparticles. In this study, we report the development of surface-enhanced Raman spectroscopy (SERS) as a method to monitor the kinetics of gold–thiolate bond formation on colloidal gold nanoparticles. A theoretical model combining SERS enhancement with the Beer–Lambert law is proposed to explain ensemble scattering and absorption effects in colloids during chemisorption. In order to maximize biological relevance and signal reproducibility, experiments used to validate the model focused on maintaining nanoparticle stability after the addition of water-soluble aromatic thiolated molecules. Our results indicate that ligand exchange on gold nanoparticles follow a first-order Langmuir adsorption model with rate constants on the order of 0.01 min−1. This study demonstrates an experimental spectroscopic method and theoretical model for monitoring binding kinetics that may prove useful for designing novel probes.
- This article is part of the themed collection: Advisory Board research selection

 Please wait while we load your content...
Please wait while we load your content...