Thermoresponsive copolymers with pendant d-galactosyl 1,2,3-triazole groups: synthesis, characterization and thermal behavior†
Abstract
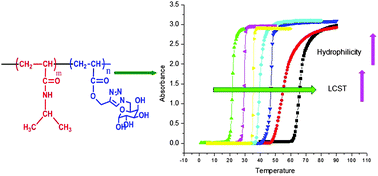
A galactose containing glycomonomer has been synthesized by copper catalyzed azide–alkyne cyclo-addition reaction (CuAAC) of 6-azido-6-deoxy-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose with propargyl acrylate. This monomer was subjected to homopolymerization and copolymerization with N-isopropylacrylamide (NIPAm) at different compositions by free radical polymerization using 2,2′-azobis-isobutyronitrile (AIBN) as an initiator. The composition of the copolymer was determined by 1H-NMR spectroscopy. Upon acid hydrolysis of acetonide protected polymers, water-soluble deprotected polymers were obtained. The polymers were characterized and confirmed by NMR, IR, GPC and thermal analytical techniques. The protected and deprotected copolymers showed a sharp cloud-point temperature and a linear correlation was obtained between the lower critical solution temperatures (LCST) and the concentration of glycomonomer in the copolymers. This allows tuning the thermal response by simply altering the copolymer composition. Water contact angle experiments showed the changes in the hydrophilicity of copolymers with compositions that supported the LCST results. The glass transition temperature of protected copolymers followed a regular monotonic decreasing trend with increasing glycomonomer content, whereas Tg of deprotected copolymers increased due to H-bond interaction. The attempts to develop thermoresponsive polymers having LCSTs at physiological temperature were successful.

 Please wait while we load your content...
Please wait while we load your content...