Computational thermodynamic study on the complexes of Am(iii) with tridentate N-donor ligands†
Abstract
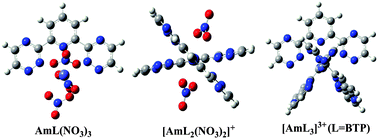
To assess the role of the lateral triazine group of 2,6-bis(1,2,4-triazin-3-yl) pyridine (BTP) when coordinated to Am(III), three tridentate N-donor ligands, i.e. BTP, 6-(-2-pyridyl)-2-pyridyl (hemi-BTP), and 2,2′:6′2′′-terpyridine (TPY), have been used to construct coordination complexes with Am(III), and the structures and binding modes of these complexes have been investigated using the B3LYP functional. The 1 : 1 and 1 : 2 (metal : ligand) type complexes, based on our calculations, form mainly via reactions Am(H2O)3(NO3)3 + L → AmL(NO3)3 + 3H2O and [Am(H2O)6(NO3)2]+ + 2L → [AmL2(NO3)2]+ + 6H2O. The Gibbs free energy changes were in the order of TPY > hemi-BTP > BTP, independent of the presence of nitrate ions in the complexes. We show that in 1 : 1 type complexes substitution of electron-donating groups to the three ligands can enhance their binding ability. From analysis of NPA charge and Mayer Bond Order, it is found that the value of binding free energy is correlated with charge transfers between the central metal and the ligand: the larger the ligand-to-metal charge transfer, the more negative the binding energy, and meanwhile, the smaller the Mayer bond order of the Am–N bonds. This suggests that the interaction between Am(III) and the tridentate ligands has a strong ionic feature, which is confirmed by the quantum theory of atoms-in-molecules (QTAIM) topological analysis. According to our calculations, the presence of the triazine group in BTP and hemi-BTP does not improve the binding affinity of the ligand to Am(III), compared to TPY, but facilitates the ligand to adopt a conformation that favors to coordinate with Am3+ than others via a dynamic isomerization process, and the electron-donating groups on the triazine group may enhance the charge transfer between Am(III) and the ligand, and thus stabilize the complex. We tentatively propose that the facile conversion between the conformations of BTP, which is more difficult for TPY and hemi-BTP, may significantly contribute to its higher affinity towards binding with Am(III).
- This article is part of the themed collection: Frontiers of Organo-f-Element Chemistry

 Please wait while we load your content...
Please wait while we load your content...