Universal degradation performance of a high-efficiency AgBr/Ag2CO3 photocatalyst under visible light and an insight into the reaction mechanism†
Abstract
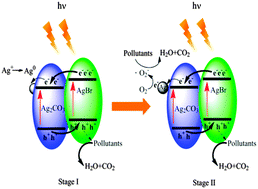
A high-efficiency visible light-driven AgBr/Ag2CO3 photocatalyst has been synthesized via a simple ion-exchange method. The textural, morphological and optical properties of the photocatalyst were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), nitrogen adsorption–desorption isothermal measurement and UV-vis diffuse reflectance spectroscopy (DRS). The prepared composite exhibited excellent photocatalytic efficiency for the degradation of methyl orange (MO), tetracycline (TC) and bisphenol A (BPA) under visible light irradiation (λ > 420 nm). Compared to pure Ag2CO3, AgBr/Ag2CO3 exhibited considerably higher photocatalytic activity and stability. The quenching effects of different scavengers and electron spin resonance (ESR) analysis suggest that holes and ˙O2− were the main reactive species responsible for the pollutant degradation and holes played the leading role. Two electron reaction processes were verified in the AgBr/Ag2CO3 photocatalytic system on the basis of ESR and X-ray photoelectron spectroscopy (XPS). Thus, a two-stage photocatalytic mechanism was proposed.

 Please wait while we load your content...
Please wait while we load your content...