A simple and effective 1,2,3-triazole based “turn-on” fluorescence sensor for the detection of anions†
Abstract
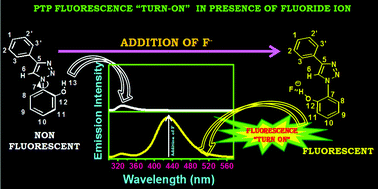
A novel and effective 1,2,3-triazole based fluorescence chemosensor has been synthesized for the specific detection of anions in homogeneous medium. Notably, the molecule, synthesized in one step using “Click chemistry”, is a simple 1,4-diaryl-1,2,3-triazole, containing a phenol moiety. The probe displayed the strongest response to fluoride ion through the “turn-on” fluorescence sensing mechanism when screened for selectivity and sensitivity against a series of anions (F−, Cl−, Br−, I−, H2PO4−, ClO4−, OAc−, BF4−). Fluorescence spectroscopy and Nuclear Magnetic Resonance Spectroscopy (NMR) studies substantiate 1 : 1 stoichiometry between the probe and fluoride anion. Kinetic studies and the single crystal X-ray spectroscopic evidence revealed the binding interaction occurs with the phenolic group and the anion.

 Please wait while we load your content...
Please wait while we load your content...