Methionine oxidation of amyloid peptides by peroxovanadium complexes: inhibition of fibril formation through a distinct mechanism†
Abstract
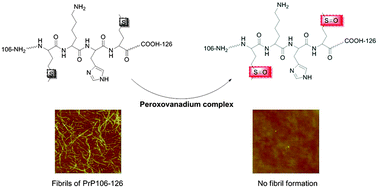
Fibril formation of amyloid peptides is linked to a number of pathological states. The prion protein (PrP) and amyloid-β (Aβ) are two remarkable examples that are correlated with prion disorders and Alzheimer's disease, respectively. Metal complexes, such as those formed by platinum and ruthenium compounds, can act as inhibitors against peptide aggregation primarily through metal coordination. This study revealed the inhibitory effect of two peroxovanadium complexes, (NH4)[VO(O2)2(bipy)]·4H2O (1) and (NH4)[VO(O2)2(phen)]·2H2O (2), on amyloid fibril formation of PrP106–126 and Aβ1–42via site-specific oxidation of methionine residues, besides direct binding of the complexes with the peptides. Complexes 1 and 2 showed higher anti-amyloidogenic activity on PrP106–126 aggregation than on Aβ1–42, though their regulation on the cytotoxicity induced by the two peptides could not be differentiated. The action efficacy may be attributed to the different molecular structures of the vanadium complex and the peptide sequence. Results reflected that methionine oxidation may be a crucial action mode in inhibiting amyloid fibril formation. This study offers a possible application value for peroxovanadium complexes against amyloid proteins.
- This article is part of the themed collection: Alzheimer's Research Month 2016

 Please wait while we load your content...
Please wait while we load your content...