Multiscale analysis of the murine intestine for modeling human diseases
Abstract
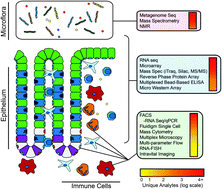
When functioning properly, the intestine is one of the key interfaces between the human body and its environment. It is responsible for extracting nutrients from our food and excreting our waste products. It provides an environment for a host of healthful microbes and serves as a first defense against pathogenic ones. These processes require tight homeostatic controls, which are provided by the interactions of a complex mix of epithelial, stromal, neural and immune cells, as well as the resident microflora. This homeostasis can be disrupted by invasive microbes, genetic lesions, and carcinogens, resulting in diseases such Clostridium difficile infection, inflammatory bowel disease (IBD) and cancer. Enormous strides have been made in understanding how this important organ functions in health and disease using everything from cell culture systems to animal models to human tissue samples. This has resulted in better therapies for all of these diseases, but there is still significant room for improvement. In the United States alone, 14 000 people per year die of C. difficile, up to 1.6 million people suffer from IBD, and more than 50 000 people die every year from colon cancer. Because these and other intestinal diseases arise from complex interactions between the different components of the gut ecosystem, we propose that systems approaches that address this complexity in an integrative manner may eventually lead to improved therapeutics that deliver lasting cures. This review will discuss the use of systems biology for studying intestinal diseases in vivo with particular emphasis on mouse models. Additionally, it will focus on established experimental techniques that have been used to drive this systems-level analysis, and emerging techniques that will push this field forward in the future.
- This article is part of the themed collection: In Vivo Systems Biology

 Please wait while we load your content...
Please wait while we load your content...