Analysis and understanding of amido black 10B dye degradation in aqueous solution by electrocoagulation with the conventional oxidants peroxomonosulfate, peroxodisulfate and hydrogen peroxide†
Abstract
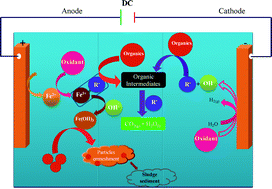
We investigated the degradation of amido black 10B dye by electrocoagulation (EC) processes assisted by the conventional oxidants peroxomonosulfate (PMS), peroxodisulfate (PDS) and hydrogen peroxide (HP). These EC processes were based on the formation of hydroxyl (HO˙) and sulfate (SO4˙−) radicals through electrochemically generated Fe2+/Fe3+-mediated activation of PMS, HP and PDS. Special attention was given to understanding the influence of the applied current, the initial solution pH and the concentration of the different oxidants on the degradation of the amido black 10B dye. The optimum operating conditions for each of these parameters was verified experimentally using Fe electrodes. Our results suggested that a low applied current and an acidic environment (pH 5) were favorable and very effective conditions for oxidant-assisted EC processes. The experimental results also showed that the oxidant-assisted EC processes produced exceptional degradation (99%) of amido black 10B dye with low energy consumption under the optimum conditions of CAB = 0.16 mM, CNaCl = 17 mM, CPMS = CPDS = 0. 16 mM, CHP = 0.13 mM and pH 5. The efficiency of degradation of amido black 10B dye by oxidant-assisted EC was in the order PMS > PDS > HP. The UV–visible spectral changes clearly showed that the azo linkage (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) was degraded more easily by oxidant-assisted EC processes than the aromatic rings of the amido black 10B dye molecule. The maximum mineralization efficiencies attained for EC and EC assisted by PMS, PDS and HP were 34, 66, 57 and 46%, respectively.
N–) was degraded more easily by oxidant-assisted EC processes than the aromatic rings of the amido black 10B dye molecule. The maximum mineralization efficiencies attained for EC and EC assisted by PMS, PDS and HP were 34, 66, 57 and 46%, respectively.

 Please wait while we load your content...
Please wait while we load your content...