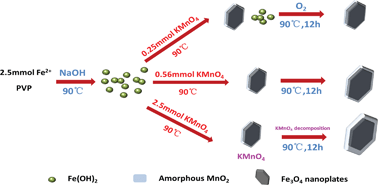
In this study, we proposed a one-pot, low-cost and environmentally-friendly approach for fabrication of tunable Fe3O4–MnO2 core–shell nanoplates at ambient temperature and pressure. The results of high resolution transmission electron microscope (HRTEM) and energy dispersive X-ray (EDX) spectra showed that Fe3O4 nanoplate cores were coated by amorphous MnO2 shells. In addition, the morphology of the as-synthesized nanoparticles could be controlled through manipulating the molar ratio of FeSO4 and KMnO4. The increased KMnO4 dosage led to the decrease in thickness of the nanoplate cores from 12 nm to <5 nm, the increase in edge length from 40 nm to 70 nm, and the increase in thickness of the shell from <1 nm to 5 nm. In addition, the formation mechanism of Fe3O4–MnO2 core–shell nanoplates was proposed based on all the observations at arbitrary molar ratio of Fe and Mn precursors. Last, the performance of sample 2 was examined as an absorbent for removal of arsenic anions from aqueous solution, and the results showed high removal capacity for both arsenite and arsenate. Moreover, the synthesized NPs have been demonstrated to be capable of enhancing the adsorption of arsenite through simultaneous oxidation of arsenite to arsenate by MnO2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...