Charge-displacement analysis as a tool to study chalcogen bonded adducts and predict their association constants in solution†
Abstract
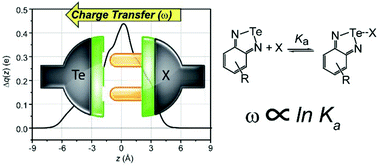
The secondary interaction between a polarized chalcogen atom and different Lewis bases, either anionic or neutral, has been studied by charge displacement analysis. Using charge displacement analysis, the charge rearrangement in the adduct upon the formation of the interaction has been quantified and described in great detail. By comparing the theoretical results with the experimental association constants, two linear correlations can be found for anionic and neutral bases. Such correlations can be used to reliably predict the association constants of adducts for which experimental data are not available yet.
- This article is part of the themed collections: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST and 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...