Aryl-BIAN-ligated silver(i) trifluoromethoxide complex†
Abstract
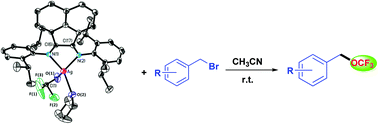
A reaction of acetonitrile-solvated AgOCF3 with 1 equiv. of Aryl-BIAN ligand in THF at room-temperature afforded the silver(I) complex (Aryl-BIAN)AgOCF3 (1) in 75% yield. The crystal structure of this silver(I) trifluoromethoxide was determined by single-crystal X-ray crystallography. The molecular structure of 1 shows the metal centre bound to one molecule of BIAN, one trifluoromethoxide and one THF solvate, resulting in a distorted tetrahedral silver. Density functional theory (DFT) calculations and the natural bond orbital (NBO) analysis were conducted to give insights into the electronic structure of 1 and the bonding characters of the OCF3 group. The reactivity of 1 towards trifluoromethoxylation of organic halides was also examined; a reaction with benzyl bromides gave the desired products of benzyl trifluoromethyl ethers in good to excellent yields.
- This article is part of the themed collection: Fluorine

 Please wait while we load your content...
Please wait while we load your content...