The structure of trimethyltin fluoride†
Abstract
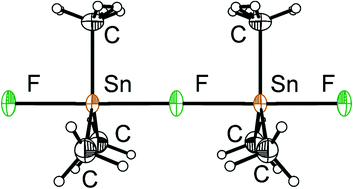
The solid-state structure of (CH3)3SnF was reinvestigated by X-ray diffraction techniques as well as by multi-nuclear solid-state NMR spectroscopy. Trimethyltin fluoride crystallizes from hot ethanol in the orthorhombic space group Pnma at room temperature and changes to a low-temperature orthorhombic phase (space group: Cmcm) below −70 °C. In both modifications, trimethyltin fluoride adopts a linear chain structure with symmetric fluorine bridges, in contrast to previous reports. During its synthesis, (CH3)3SnF precipitates in another, poorly crystalline modification, as shown by powder X-ray diffraction. Solid-state MAS NMR experiments of both room-temperature phases of (CH3)3SnF (non-recrystallized and recrystallized) were carried out for the 1H, 13C, 19F, and 119Sn nuclei. The 119Sn{19F, 1H} and 19F{1H} NMR spectra offer unambiguous determination for the 19F and 119Sn shielding tensors. The 119Sn{1H} solid-state NMR spectra are in agreement with pentacoordination of Sn in this compound for the non-recrystallized and the recrystallized modifications. Based on the solid-state NMR results, the non-recrystallized modification of (CH3)3SnF also consists of linear, symmetrically fluorine-bridged chains, and differs from the recrystallized orthorhombic phase only in packing of the chains.
- This article is part of the themed collection: Fluorine

 Please wait while we load your content...
Please wait while we load your content...