What causes the weakest host to act as the strongest one? A theoretical study on the host–guest chemistry of five azacryptands and fluoride anions†
Abstract
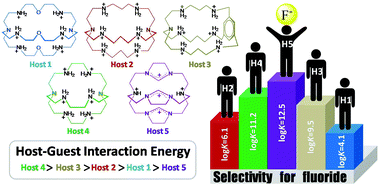
In this work we have attempted to computationally analyze the important parameters affecting the selectivity in host–guest systems in order to show that the solvent effect in some host–guest systems is even more important than the hole-size fitting and/or host–guest interaction energy. For this purpose, the fluoride anion selectivities of the five most studied azacryptands with different affinities, moieties, cavity sizes and degrees of preorganization, in their fully protonated forms, were studied at PBE/TZVP and B3P86/TZVP levels of theory. The factors affecting the selectivity such as hole size fitting, steric effects, electronic properties, preorganization of hosts, as well as solvent effects were investigated. The results showed that among the five studied azacryptands, one of them, which ranks fifth for selectivity according to its weak interaction energy with fluoride anions, ranks fourth due to its good preorganization. However, a surprising improvement occurs when its selectivity for fluoride anions ranks first due to the existence of less solvent hindrance. The results of the calculations were further confirmed by a good correlation between the calculated and experimental formation constants.
- This article is part of the themed collection: Fluorine

 Please wait while we load your content...
Please wait while we load your content...