BaCoO2.22: the most oxygen-deficient certified cubic perovskite
Abstract
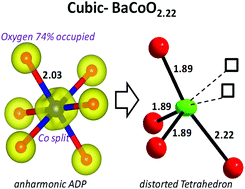
The cubic BaCoO∼2.2 was announced in the early 50's as the final product of high temperature self-reduction within the BaCoO3−δ series. However, apart from this report no clear characterization has been provided to date. Here, we confirm after the preparation of single crystal and powder samples that in this compound the ratio of oxygen vacancies is close to 27% in absence of any long range ordering. It follows that BaCoO2.22 appears as the most oxygen deficient cubic perovskite stabilized at room temperature, its tolerance factor being displaced close to 1 by the combination of large Ba2+ and Co2/3+ ions in the A and B sites. The tolerance factor plays a limiting role for re-oxidation and fluorination using topochemical routes, despite the high concentration of available vacancies. Single crystal XRD data and DFT structural relaxation show that the Co sites are off-centered inside pseudo-tetrahedra leading to reinforced magnetic exchanges. Robust antiferromagnetic ordering is suggested to occur above 400 K while this compound shows a semi-conducting behavior. It was also possible to prepare an even more reduced mixed metallic phase of formula BaCo0.5Fe0.5O2.16.
- This article is part of the themed collection: Perovskites

 Please wait while we load your content...
Please wait while we load your content...