Sonochemical redox reactions of Pu(iii) and Pu(iv) in aqueous nitric solutions†
Abstract
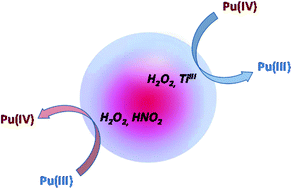
The behavior of Pu(IV) and Pu(III) was investigated in aqueous nitric solutions under ultrasound irradiation (Ar, 20 kHz). In the absence of anti-nitrous reagents, ultrasound has no effect on Pu(IV), while Pu(III) can be rapidly oxidized to Pu(IV) due to the autocatalytic formation of HNO2 induced by HNO3 sonolysis. In the presence of anti-nitrous reagents (sulfamic acid or hydrazinium nitrate), Pu(IV) can be sonochemically reduced to Pu(III). The reduction follows a first order reaction law and leads to a steady state where Pu(IV) and Pu(III) coexist in solution. The reduction process is attributed to the sonochemical generation of H2O2 in solution. The kinetics attributed to the reduction of Pu(IV) are however higher than those related to the formation of H2O2 which, after several hypotheses, is explained by the sonochemical erosion of the titanium-based sonotrode. Titanium particles thereby generated can be solubilized under ultrasound and generate Ti(III) as an intermediate species, a strong reducing agent able to react with Pu(IV).
- This article is part of the themed collection: Dalton Discussion 14: Advancing the chemistry of the f-elements

 Please wait while we load your content...
Please wait while we load your content...