Hydration of the sulfate dianion in cold nanodroplets: SO42−(H2O)12 and SO42−(H2O)13†
Abstract
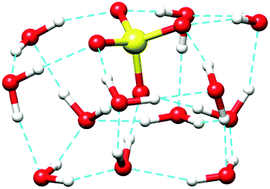
The structures, energetics and infrared spectra of SO42−(H2O)12 and SO42−(H2O)13 have been investigated by a combination of classical polarizable molecular dynamics and static quantum chemical calculations. Snapshots extracted from MD trajectories were used as inputs for local DFT optimization. Energies of the most stable structures were further refined at the ab initio level. A number of new low energy structures have thus been identified. The most stable structures of SO42−(H2O)12 have the sulfate on the surface of the water cluster, while it may be slightly more burried in SO42−(H2O)13, however still with an incomplete first hydration shell. Differences in the infrared spectra arise in part from mixing of sulfate stretching and water librational modes in the 900–1100 cm−1 region, leading to some sensitivity of the IR spectrum to the structure. Second shell water molecules however do not generate signatures that are specific enough to relate spectra to structures straightforwardly, at least in this frequency range. Thus the emergence of a new band at 970 cm−1 in the SO42−(H2O)13 spectrum cannot be taken as a clue as to the number of water molecules which is necessary for a cluster to close the first hydration shell of sulfate. This number is at least 14 and possibly larger. However the density of low energy isomers is large enough that individual structures may loose meaning at all but the lowest temperatures.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...