Photoisomerization action spectroscopy: flicking the protonated merocyanine–spiropyran switch in the gas phase†
Abstract
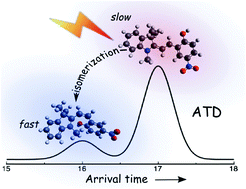
Laser spectroscopy and ion mobility spectrometry are combined to provide structural and photochemical information on photoisomerizing molecules in the gas phase. The strategy exploits the fact that an ion packet propelled through buffer gas by an electric field separates spatially and temporally into its constituent isomers because of small differences in their collision cross sections. Isomers selected by an electrostatic ion gate are exposed to wavelength tunable radiation, promoting formation of photoisomers that are separated in a second ion mobility stage. The approach is demonstrated for protonated merocyanine and spiropyran isomers formed through electrospray ionization. Four isomers are observed whose relative abundances depend on pretreatment of the electrosprayed solution with either ultraviolet or visible light, and on collisional excitation before the ions are launched into the drift tube. The observations are interpreted in the light of accurate double-hybrid density functional theory calculations for the protonated spiropyran and merocyanine isomers that are used to predict structures, relative energies, isomerization barriers, collision cross sections and electronic absorption spectra. The two most abundant isomers, are merocyanine forms, in which the proton resides on the quinone oxygen atom, with either a trans or cis central bond in the linking polymethine chain. These two mero forms can be interconverted through photoexcitation, with different wavelength dependences for the forward and reverse photoisomerization processes. Protonated spiropyran is formed from protonated merocyanine isomers through collisional activation, but in only minor amounts through their photo-excitation over the 300–700 nm range.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods


 Please wait while we load your content...
Please wait while we load your content...