Synergistic effects of Ir–Au/TiO2 catalysts in the total oxidation of propene: influence of the activation conditions†
Abstract
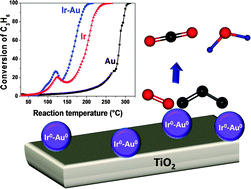
Iridium was added to the Au/TiO2 system to try to enhance its catalytic activity in the reaction of propene oxidation, performed under conditions close to those used in the studies of decomposition of volatile organic compounds (1200 ppm propene and 9 vol% O2 in He). Titania supported Ir–Au (Ir/Au = 1) was prepared by sequential deposition–precipitation with urea (DPU) of Ir then Au. The effect of the activation conditions (hydrogen or air at 400 °C) was investigated. The study of the activation conditions of Ir–Au/TiO2 showed that activation under hydrogen at 400 °C generated a catalyst more active than the monometallic ones, while Ir–Au/TiO2 activated in air remained as poorly active as Au/TiO2. TEM characterization showed the formation of metallic particles of similar size (2–3 nm) in both monometallic Au/TiO2 and bimetallic Ir–Au/TiO2. Characterization especially by DRIFTS using CO as a probe molecule suggests the presence of Ir–Au interaction, IrO2–Au0 interaction when the sample is calcined and Ir0–Au0 bimetallic particles when it is reduced. XPS and TPR characterization techniques showed that gold hinders to some extent the reoxidation of iridium in the reduced bimetallic Ir–Au/TiO2 catalyst. The enhanced catalytic activity of the reduced bimetallic Ir–Au/TiO2 catalyst is attributed to a surface Ir0–Au0 synergism.
- This article is part of the themed collection: Recent advances in the chemical physics of nanoalloys

 Please wait while we load your content...
Please wait while we load your content...