Specific adhesion of membranes simultaneously supports dual heterogeneities in lipids and proteins†
Abstract
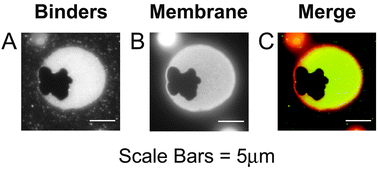
Membrane adhesion is a vital component of many biological processes. Heterogeneities in lipid and protein composition are often associated with the adhesion site. These heterogeneities are thought to play functional roles in facilitating signalling. Here we experimentally examine this phenomenon using model membranes made of a mixture of lipids that is near a phase boundary at room temperature. Non-adherent model membranes are in a well-mixed, disordered-fluid lipid phase indicated by homogeneous distribution of a fluorescent dye that is a marker for the fluid-disordered (Ld) phase. We specifically adhere membranes to a flat substrate bilayer using biotin–avidin binding. Adhesion produces two types of coexisting heterogeneities: an ordered lipid phase that excludes binding proteins and the fluorescent membrane dye, and a disordered lipid phase that is enriched in both binding proteins and membrane dye compared with the non-adhered portion of the same membrane. Thus, a single type of adhesion interaction (biotin–avidin binding), in an initially-homogeneous system, simultaneously stabilizes both ordered-phase and disordered-phase heterogeneities that are compositionally distinct from the non-adhered portion of the vesicle. These heterogeneities are long-lived and unchanged upon increased temperature.
- This article is part of the themed collection: Chemical compartmentalisation by membranes: from biological mechanism to biomimetic applications

 Please wait while we load your content...
Please wait while we load your content...