Separating mixtures by exploiting molecular packing effects in microporous materials†
Abstract
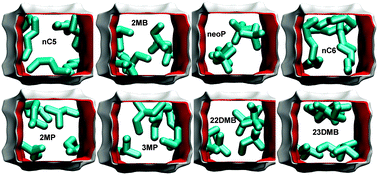
We examine mixture separations with microporous adsorbents such as zeolites, metal–organic frameworks (MOFs) and zeolitic imidazolate frameworks (ZIFs), operating under conditions close to pore saturation. Pore saturation is realized, for example, when separating bulk liquid phase mixtures of polar compounds such as water, alcohols and ketones. For the operating conditions used in industrial practice, pore saturation is also attained in separations of hydrocarbon mixtures such as xylene isomers and hexane isomers. Separations under pore saturation conditions are strongly influenced by differences in the saturation capacities of the constituent species; the adsorption is often in favor of the component with the higher saturation capacity. Effective separations are achieved by exploiting differences in the efficiency with which molecules pack within the ordered crystalline porous materials. For mixtures of chain alcohols, the shorter alcohol can be preferentially adsorbed because of its higher saturation capacity. With hydrophilic adsorbents, water can be selectively adsorbed from water–alcohol mixtures. For separations of o-xylene–m-xylene–p-xylene mixtures, the pore dimensions of MOFs can be tailored in such a manner as to allow optimal packing of the isomer that needs to be adsorbed preferentially. Subtle configurational differences between linear and branched alkane isomers result in significantly different packing efficiencies within the pore topology of MFI, AFI, ATS, and CFI zeolites. A common characteristic feature of most separations that are reliant on molecular packing effects is that adsorption and intra-crystalline diffusion are synergistic; this enhances the separation efficiencies in fixed bed adsorbers.

 Please wait while we load your content...
Please wait while we load your content...