Synthesis and biological evaluation of pharbinilic acid and derivatives as NF-κB pathway inhibitors†
Abstract
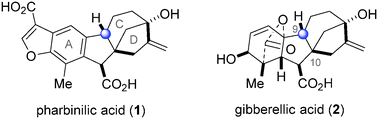
A 7-step synthesis of pharbinilic acid, a member of the gibberellin family of natural products and the first naturally occurring allogibberic acid, is reported. An efficient decarboxylative aromatization reaction enables the synthesis of pharbinilic acid and related analogs for evaluation as modulators of NF-κB activity. Remarkably, one analog displays a 2 μM IC50 in an NF-κB activity assay and inhibits an endogenous NF-κB-regulated pathway.

 Please wait while we load your content...
Please wait while we load your content...