Complete switch from α-2,3- to α-2,6-regioselectivity in Pasteurella dagmatis β-d-galactoside sialyltransferase by active-site redesign†
Abstract
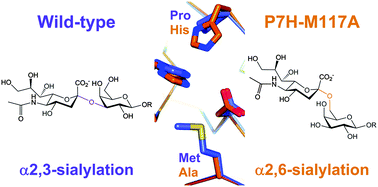
Structure-guided active-site redesign of a family GT-80 β-D-galactoside sialyltransferase (from Pasteurella dagmatis) to change enzyme regioselectivity from α-2,3 in the wild type to α-2,6 in a P7H–M117A double mutant is reported. Biochemical data for sialylation of lactose together with protein crystal structures demonstrate highly precise enzyme engineering.

 Please wait while we load your content...
Please wait while we load your content...