C-axis preferentially oriented and fully activated TiO2 nanotube arrays for lithium ion batteries and supercapacitors†
Abstract
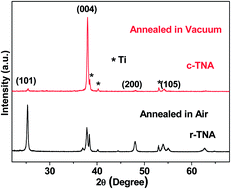
We report the fabrication of long titanium dioxide nanotube arrays with highly c-axis preferentially oriented crystallization and a high concentration of oxygen vacancies by second anodization in ethylene glycol and annealing under poor-oxygen conditions. By optimizing the growth and annealing conditions, the [001] oriented crystallization is maximized, and 31.7% of the total Ti ions exists as Ti3+ ions. The carrier density of the [001]-oriented TiO2 nanotube arrays is two orders of magnitude higher than that of the randomly oriented TiO2 nanotube arrays. The unusual c-textured crystallization confined within nanotubes may involve the formation of TiO62− octahedra with a gradient distribution along the tube axis, preferential nucleation at the top, and preferential growth downwards along the c axis. Because of the c-axis preferential orientation and a high-concentration of oxygen vacancies, long TiO2 nanotube arrays can serve as superior electrodes for both lithium ion batteries and supercapacitors without the addition of any conductive agents. Long c-oriented TiO2 nanotube arrays deliver reversible capacities of 293 mA h g−1 at 0.5 C and 174 mA h g−1 at 5 C with Coulombic efficiencies of over 99%, and hold an areal capacitance of 8.21 mF cm−2 with an 85% capacitance retention after 5000 cycles. Double roles played by oxygen vacancies are identified in increasing electrical conductivity and activating the rich-Li phase.
- This article is part of the themed collection: 2014 Journal of Materials Chemistry A Hot Articles

 Please wait while we load your content...
Please wait while we load your content...