Structural re-arrangement in two hexanuclear CuII complexes: from a spin frustrated trigonal prism to a strongly coupled antiferromagnetic soluble ring complex with a porous tubular structure†
Abstract
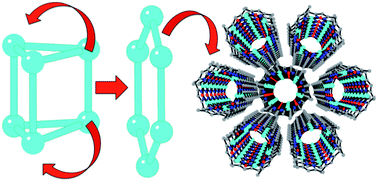
The addition of water to a chloroform solution of the Cu6 trigonal prism complex [Cu6(μ6F)(μ2OH)(μ3OCH3)2(μ2OCH3)2(3,5-Me2pz)6] (1) (3,5-Me2pz− = 3,5-dimethylpyrazolate) results in the formation of the Cu6 planar hexagonal ring complex [Cu6(μ2OH)6(3,5-Me2pz)6]·CH3CN·CHCl3 (2). A simple mechanism for this structural re-arrangement is proposed, in which 2 can be viewed as a hydrolysis product of 1. This process is clearly noticeable in the magnetic properties, which change from spin frustrated with a weak antiferromagnetic coupling in 1, to strongly antiferromagnetic in 2. Interestingly, the hexagonal ring complex 2 self-assembles in the solid state to form a porous hexagonal tubular structure containing guest solvent molecules that can be removed and CO2-exchanged without loss of crystallinity.

 Please wait while we load your content...
Please wait while we load your content...