Self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of arteether: pharmacokinetics, toxicity and antimalarial activity in mice
Abstract
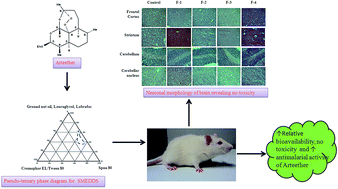
The aim of the study was to develop oral arteether (AE) nano formulations, to investigate their effects in rats, and for the complete and effective treatment of Plasmodium yoelii nigeriensis infected mice at a reduced dose by increasing the relative bioavailability. Nano-formulations of arteether have been developed. The relative bioavailability (RB%) was assessed by calculating individual Area under curve (AUC) AUC0–t, AUC0–∞ and Cmax values. Haematological and biochemical parameters were estimated in rats and sections of brain and peripheral organs were analyzed for histopathological changes. The formulations were tested for antimalarial efficacy and safety in Plasmodium yoelii nigeriensis infected swiss mice. The AUC for lipid formulations (AUC0–t 4.98 ± 0.79 h μg ml−1) and AUC0–∞ (5.02 ± 0.80 h μg ml−1) was significantly higher (p < 0.05) than for AE in ground nut oil (GNO) and AE in aqueous suspension. The Cmax was also significantly higher for all the formulations. The RB% has been found to be significantly higher (257%) in the formulations than for AE in GNO. No considerable changes have been monitored in the serum biochemical parameters of rats. These formulations have been found to be highly effective for the treatment of Plasmodium yoelii nigeriensis infected swiss mice, even at the lower dose of 12.5 mg kg−1 × 5 days. Overall the developed formulations are safe, provide a non-toxic platform for further clinical studies, and can be used in artemisinin-based combination therapies (ACTs).

 Please wait while we load your content...
Please wait while we load your content...