Bioinspired synthesis of mesoporous ZrO2 nanomaterials with elevated defluoridation performance in agarose gels
Abstract
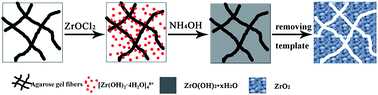
Mesoporous ZrO2 nanomaterials were synthesized in an agarose gel medium by a bioinspired approach, and their adsorption capacity for the fluoride anion was also evaluated. The obtained ZrO2 samples after calcination at 600 °C for 2 h are primarily composed of tetragonal nanocrystals about 11.8 nm in size. Moreover, the samples possess bimodal mesopores about 3.5 nm and 5.9 nm in diameter, which are generated by removing the agarose gel fibers embedded in the ZrO2 particles during their growth process. The pore volume and specific surface area of mesoporous ZrO2 nanomaterials are 0.091 cm3 g−1 and 40.14 m2 g−1, respectively, which increase with the increase of gel concentration, while decrease with the increase of initial ZrOCl2 concentration. The ZrO2 samples synthesized in agarose gel show a higher fluoride adsorption capacity than those synthesized in the aqueous solution by the same method. This study may provide a broader insight into the bioinspired synthesis of metal oxides in hydrogel medium under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...