Evidence supporting a 1,2-dioxetanone as an intermediate in the benzofuran-2(3H)-one chemiluminescence†
Abstract
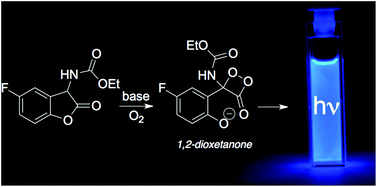
The mechanism of the chemiluminescent reaction of ethyl (5-fluoro-2-oxo-2,3-dihydrobenzofuran-3-yl) carbamate (a 2-coumaranone derivative) with a base and molecular oxygen was investigated. New evidence from the reaction kinetics and absorption/emission profiles was obtained, supporting the existence of a 1,2-dioxetanone as an intermediate: (i) its characteristic activation parameters (ΔH≠ = 7.2 ± 0.1 kcal mol−1; ΔS≠ = −45 ± 5 cal K−1 mol−1) indicating a high degree of thermal instability and (ii) its bimolecular decomposition rate constant for the reaction with perylene. The newly developed methodology has been shown to be suitable for determining the reactivity of such thermally unstable peroxides, which are very difficult to prepare and isolate, using this alternative approach of in situ generation of a 1,2-dioxetanone.

 Please wait while we load your content...
Please wait while we load your content...