A total synthesis of (+)-negamycin through isoxazolidine allylation†
Abstract
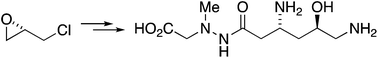
The β-amino acid antibiotic (+)-negamycin has been synthesised in ten steps from epichlorohydrin via Sakurai allylation of an isoxazolidine intermediate. The key allylation reaction proceeded with complete trans-selectivity, which is attributed to electrostatic attraction between the chlorine atom and the iminium ion in the Sakurai intermediate.
- This article is part of the themed collection: In Celebration of Richard Taylor’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...