Crosstalk between kinases and Nedd4 family ubiquitin ligases
Abstract
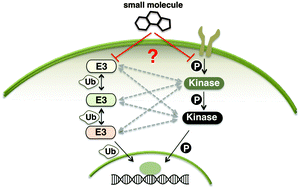
A dazzling array of human biological processes achieves coordination and balance through the posttranslational modification of protein residues with phosphate (95 Da) or ubiquitin (8565 Da). Over the past years, a reciprocal communication has become recognized between phosphorylating (kinases) and ubiquitinating (E3 ligases) enzymes. Such crosstalk occurs when a kinase acts on a ligase or vice versa to modify the catalytic activity, substrate specificity, or subcellular localization of the modified enzyme. In this review, we focus on the crosstalk between the nine members of the Nedd4 family E3 ubiquitin ligases with kinase signal transducers such as cell surface receptors, cytosolic kinases, phosphatases, and transcription factors. Since protein kinases are well explored and established therapeutic targets, we hypothesize that mapping E3 ligases onto kinase signalling networks will provide clues to the full therapeutic potential of pharmacologically targeting E3 ligases.
- This article is part of the themed collection: 2014 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...