Quantitative phosphoproteomic profiling of PINK1-deficient cells identifies phosphorylation changes in nuclear proteins†
Abstract
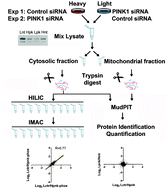
The Parkinson's disease (PD) associated gene PINK1 encodes a protein kinase that mediates the phosphorylation of multiple proteins involved in mitochondrial homeostasis. The broader downstream signaling events mediated by PINK1 kinase activity have not been well documented. We combine quantitative phosphoproteomic strategies with siRNA mediated PINK1 knock down in mammalian cells to identify alterations of phosphorylation events downstream of PINK1. Although down-regulation of PINK1 has no major effect on the proteome expression in these cells, phosphorylation of over one hundred proteins was reduced reflecting basal levels of phosphorylation signaling events downstream of PINK1. Motif analysis of the residues flanking the phosphorylation sites indicates proline-directed kinase specificity. Surprisingly, we found that the downstream signaling nodes included many transcription factors, as well as nuclear proteins involved in DNA and RNA metabolism. Thus, PINK1 dependent phosphorylation signaling may regulate nuclear activities.
- This article is part of the themed collection: 2014 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...