Digestive diversity and kinetic intrigue among heated and unheated β-lactoglobulin species†
Abstract
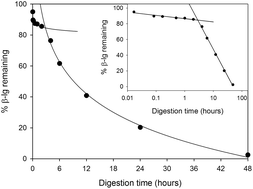
Food processing often alters the structure of proteins, and proteins are deliberately denatured and aggregated to improve technological functionality in many cases. However, the digestive consequences of processing-induced alterations to protein structure have only recently been studied. Here we explored the process–structure–digestibility relationship in the context of heat-processing effects on the structure and gastric digestibility of the bovine whey protein β-lactoglobulin (β-lg). Heating β-lg produces an array of non-native monomers, dimers and aggregates, and we have characterised these with reverse-phase high performance liquid chromatography (RP-HPLC) as a complement to our earlier work using polyacrylamide gel electrophoresis (PAGE) techniques. Using a combination of SDS-PAGE and RP-HPLC we have identified pepsin-resistant dimers and peptides that appear early in digestion. In an unexpected finding, native β-lg underwent complete hydrolysis during prolonged incubation (48 h) with pepsin. Two phases of hydrolysis were identified, and the transition between phases appears to result from alterations to the secondary structure of β-lg at 3–4 h, as measured with circular dichroism spectroscopy, and/or the binding and release of a pepsin inhibitor peptide. This work has unpacked some of the complexities of the processing–structure–digestibility relationship in a highly simplified system; further work is needed to explore the implications of these findings for food processors, regulatory authorities and consumers.
- This article is part of the themed collection: Food Structures, Digestion and Health International Conference

 Please wait while we load your content...
Please wait while we load your content...