One electron oxidation potential as a predictor of rate constants of N-containing compounds with carbonate radical and triplet excited state organic matter†
Abstract
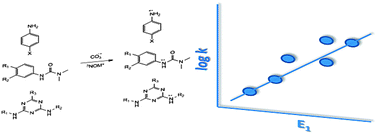
Photo-generated transient species, such as the carbonate radical and triplet excited state natural organic matter, mediate the oxidation of pollutants in various sunlit or artificially irradiated systems. In this work, one-electron oxidation potentials for 70 nitrogen-containing compounds were computed, and literature data were used to develop quantitative structure–activity relationships (QSARs) for prediction of the second order reaction rate constants with these two oxidants. For carbonate radical, separate QSARs were necessary for compounds with and without resonance stabilization of the resulting radical, and predicted rate constants were, on average, within a factor of three of experimental values. With the limited data set available, results suggest that one-electron oxidation potential is also a viable descriptor variable for predictions of rate constants with triplet excited states.
- This article is part of the themed collection: Aquatic photochemistry

 Please wait while we load your content...
Please wait while we load your content...