First- and second-sphere contributions to Fe(ii) site activation by cosubstrate binding in non-heme Fe enzymes†
Abstract
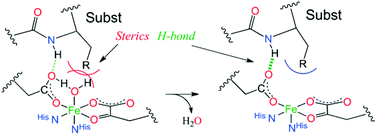
Non-heme Fe(II) enzymes exhibit a general mechanistic strategy where binding all cosubstrates opens a coordination site on the Fe(II) for O2 activation. This study shows that strong-donor ligands, steric interactions with the substrate and second-sphere H-bonding to the facial triad carboxylate allow for five-coordinate site formation in this enzyme superfamily.
- This article is part of the themed collection: Biological oxidation reactions: mechanisms and design of new catalysts

 Please wait while we load your content...
Please wait while we load your content...