Hyperpolarisation through reversible interactions with parahydrogen†
Abstract
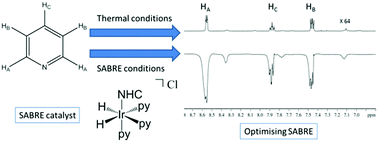
We describe here how the complexes Ir(COD)(NHC)Cl [NHC = IMes, SIMes, IPr, SIPr, ICy, IMe and ImMe2NPri2] provide significant insight into the catalytic process that underpins the hyperpolarization method signal amplification by reversible exchange (SABRE). These complexes react with pyridine and H2 to produce [Ir(H)2(NHC)(py)3]Cl which undergo ligand exchange on a timescale commensurate with good catalytic activity for the signal amplification by reversible exchange effect. This activity results from hydride ligand magnetic inequivalence and is highly dependent on the NHC. Variable temperature and kinetic studies demonstrate that rates of ligand loss which lie between 0.1 and 0.5 s−1 are ideal for catalysis. A role for the solvent complex [Ir(H)2(MeOH)(NHC)(py)2]Cl, which contains chemically inequivalent hydride ligands is revealed in the ligand exchange pathway. By optimisation of the conditions and NHC, a 5500-fold total pyridine signal enhancement is revealed when the NHC is IMes. Both T1-reduction effects and HD exchange with the solvent are probed and shown to link to catalyst efficiency. The resulting signal enhancements suggest future in vivo MRI measurements under physiological conditions using this catalytic effect will be possible.
- This article is part of the themed collection: Mechanistic Studies in Catalysis

 Please wait while we load your content...
Please wait while we load your content...